Forkortet produktinformation for >Adzynma< ® (rADAMTS13) 
▼Dette lægemiddel er underlagt supplerende overvågning. Dermed kan nye sikkerhedsoplysninger hurtigt tilvejebringes. Sundhedspersoner anmodes om at indberette alle formodede bivirkninger til Lægemiddelstyrelsen, Axel Heides Gade 1, DK-2300 København S, www.meldenbivirkning.dk.
Se venligst det fuldstændige produktresume før receptudstedelse.
Lægemiddelform: Pulver og solvens til injektionsvæske, opløsning
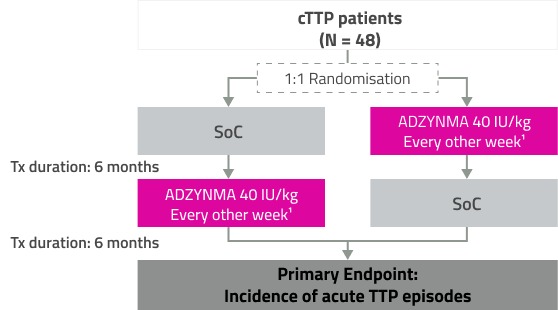
Indikation: ADZYNMA er en enzymerstatningsterapi (ERT), som er indiceret til behandling af ADAMTS13-mangel hos pædiatriske- og voksne patienter med medfødt trombotisk trombocytopenisk purpura (cTTP). ADZYNMA kan anvendes til alle aldersgrupper.
Dosering: Behandling med ADZYNMA bør påbegyndes under opsyn af en læge med erfaring i behandling af patienter med hæmatologiske sygdomme. Profylaktisk enzymerstatningsterapi: 40 IE/kg kropsvægt én gang hver anden uge. Den profylaktiske doseringshyppighed kan justeres til 40 IE/kg kropsvægt én gang om ugen baseret på klinisk respons. Behovsbaseret enzymerstatningsterapi til akutte TTP-episoder: 40 IE/kg kropsvægt på dag 1. 20 IE/kg kropsvægt på dag 2. 15 IE/kg kropsvægt fra dag 3, én gang dagligt indtil to dage efter, at den akutte hændelse er ophørt. Ældre samt ved nedsat nyre- og leverfunktion: Ikke behov for dosisjustering. Pædiatrisk population: Hos spædbørn < 10 kg legemsvægt kan der oftere være behov for at justere dosisfrekvensen fra en dosis hver anden uge til en ugentlig dosis. Administration: Kun til intravenøs anvendelse efter rekonstitution. Hjemme- eller selvadministration under opsyn af en sundhedsperson kan overvejes for patienter, der tolererer deres injektioner godt. Denne beslutning bør træffes efter evaluering og anbefaling fra den behandlende læge. Se pkt. 6.6 i det fulde produktresume for instruktioner om rekonstitution af lægemidlet før administration.
Kontraindikationer: Livstruende overfølsomhed over for det aktive stof eller over for et eller flere af hjælpestofferne.
Særlige advarsler og forsigtighedsregler: Overfølsomhedsreaktioner: Allergisk overfølsomhed, herunder anafylaktiske reaktioner, kan forekomme. Patienterne skal informeres om de tidlige tegn på overfølsomhedsreaktioner, herunder, men ikke begrænset til, takykardi, trykken for brystet, hvæsende vejrtrækning og/eller akut vejrtrækningsbesvær, hypotension, generaliseret urticaria, pruritus, rinokonjunktivitis, angioødem, letargi, kvalme, opkastning, paræstesi, rastløshed, som kan udvikle sig til anafylaktisk chok. Hvis der opstår tegn og symptomer på alvorlige allergiske reaktioner, skal administrationen af dette lægemiddel straks afbrydes, og der skal gives passende understøttende behandling. Immunogenicitet: Som med alle terapeutiske proteiner er der en risiko for immunogenicitet. Patienter kan udvikle antistoffer mod rADAMTS13 efter behandling med ADZYNMA, hvilket potentielt kan resultere i en nedsat respons på rADAMTS13 (se pkt. 5.1). Hvis der er formodning om sådanne antistoffer, og der er manglende virkning, skal man overveje andre terapeutiske strategier.
Interaktion: Der er ikke udført interaktionsstudier.
Fertilitet, graviditet og amning: Fertilitet: Der foreligger ingen data. Graviditet: Der er ingen eller utilstrækkelige data. Brug af ADZYNMA under graviditet må kun overvejes efter en grundig individuel analyse af fordele og risici foretaget af den behandlende læge både før og under behandlingen. Amning: Data for udskillelse af rADAMTS13 i human mælk eller mælk hos dyr er utilstrækkelige, men det er usandsynligt, at det udskilles i human mælk på grund af dets høje molekylvægt. Beslutningen om enten at ophøre med at amme eller stoppe behandlingen med ADZYNMA bør tage højde for, hvor vigtigt dette lægemiddel er for moderen.
Trafikfarlighed: rADAMTS13 påvirker muligvis i mindre grad evnen til at føre motorkøretøj og betjene maskiner. Der kan forekomme svimmelhed og somnolens efter administration af ADZYNMA.
Bivirkninger:
Meget almindelige: Øvre luftvejsinfektion, hovedpine, svimmelhed, migræne, diarré og kvalme.
Almindelige: Trombocytose, somnolens, forstoppelse, maveudspiling, asteni, hedeture og unormal ADAMTS13-aktivitet.
For ADZYNMA findes der ingen bivirkninger, som er ikke almindelige, sjældne, meget sjældne, eller som har ukendt hyppighed.
Indberetning af formodede bivirkninger: Axel Heides Gade 1, DK-2300 København S, Websted: www.meldenbivirkning.dk
Formodede bivirkninger bedes også rapporteret til AE.DNK@takeda.com
Overdosering: I kliniske studier blev der anvendt enkeltdoser på op til 160 IE/kg, og deres sikkerhedsprofiler var generelt i overensstemmelse med resultaterne fra kliniske studier i cTTP-patienter. I tilfælde af overdosering, baseret på den farmakologiske virkning af rADAMTS13, er der potentiale for øget risiko for blødning.
Pakninger, Priser, Udlevering og Tilskud: Der henvises til dagsaktuel pris på:
www.medicinpriser.dk
Indehaver af markedsføringstilladelsen: Takeda Manufacturing Austria AG
De med * mærkede afsnit er omskrevet/forkortet til det af EMA/Lægemiddelstyrelsens senest godkendte produktresume, som i sin helhed vederlagsfrit kan rekvireres fra Takeda Pharma A/S, Delta Park 45, 2665 Vallensbæk Strand, Tlf: 4677 1010, e-mail: medinfoemea@takeda.com
19. september 2024